
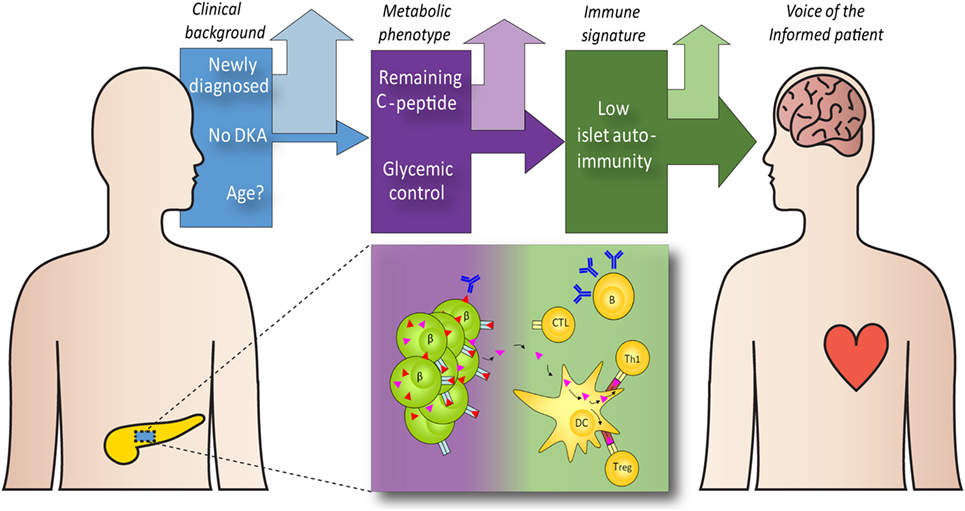
There is likely to be a fair degree of patient selection in these retrospective studies and so it is debatable how generalizable these findings are to all patients with myeloma kidney who may otherwise be transplant candidates. 80 Subsequent retrospective studies have suggested that the standard melphalan dose of 200 mg/m 2 can be used in patients with a creatinine clearance greater than 30 mL/min whereas those with creatinine clearance less than 30 mL/min or on dialysis should be treated with a dose of 140 mg/m 2. An initial report found a melphalan dose of 140 mg/m 2 reduced toxicity without compromising efficacy among 21 patients with a creatinine greater than 2 mg/dL. Because of melphalan’s predominant renal clearance, 79 the standard 200 mg/m 2 dose results in excess nonhematologic toxicity in patients with renal impairment. Transplant related mortality ranges from 0% to 38% in this setting however, the most recent and largest study from an international transplant registry found a transplant related mortality rate of 0%, for transplants performed in severe renal impairment, including patients on dialysis at the time of transplantation. In retrospective studies, it appears that transplantation is associated with an increased risk of morbidity and mortality in patients with renal impairment compared with patients with normal renal function. To date, none of these studies has reported outcomes specifically for the subgroup of patients with reduced creatinine clearance, and thus we have no high-level evidence to support the use of autologous stem cell transplantation in patients with renal impairment. In the three most recent randomized studies, the lower limit of allowed renal function was a creatinine clearance of 15, 20, and 50 mL/min, respectively. 76–78 These trials excluded patients over 65 years of age, as well as patients with more severe degrees of renal impairment. 75 Such transplants were initially shown to improve myeloma control and overall survival when compared with multiagent chemotherapy and, more recently, has been shown to provide the best long-term disease control in randomized comparisons to bortezomib, lenalidomide, or combined bortezomib-lenalidomide regimens. Several different conditioning regimens and doses have been compared over time, but intravenous melphalan at a dose of 200 mg/m 2 has been confirmed as the optimal conditioning before stem cell reinfusion, in patients with normal renal function. HUTCHISON, PETER MOLLEE, in Onco-Nephrology, 2020 Autologous stem cell transplantation in patients following a presentation with myeloma kidneyĪutologous stem cell transplantation, following high-dose melphalan, is standard consolidation therapy in younger patients with myeloma. The benefit of a second ASCT is limited to patients in whom complete response or very good partial response (> 90% reduction in M-protein level) was not achieved with the first procedure. Tandem (double) ASCT patients receive a second planned ASCT after recovery from the first transplant. Progression-free survival was longer, and the median overall survival was increased by approximately 1 year in the transplant group. In two studies from France and the UK, ASCT was superior to combination conventional chemotherapy. Melphalan (Alkeran) 200 mg/m 2 is the most widely used preparative regimen for ASCT. 22 Alkylating agents must be avoided prior to stem cell collection because they may damage stem cells. Those considered eligible for ASCT receive four cycles of induction therapy consisting of lenalidomide and weekly dexamethasone or bortezomib and dexamethasone.
Patients with renal failure may undergo ASCT but the morbidity and mortality is higher. In general, ASCT is offered to patients 2.5 mg/dL, ECOG performance status grade 3 or 4 or New York Heart Association functional status Class III or IV are generally ineligible for ASCT. Kyle, Angela Dispenzieri, in Clinical Immunology (Fourth Edition), 2013 Initial therapy for transplant eligible patientsĮligibility for ASCT varies from country to country.


 0 kommentar(er)
0 kommentar(er)
